Hydrogen A to Z Series: O for Orthohydrogen
By: GenH2 Staff
Read Time: 3 minutes
Defining The Hydrogen Economy from A to Z: O is for Orthohydrogen
Continuing in our Defining Hydrogen series, today we discuss orthohydrogen, the more stable form of hydrogen at and above room temperature, whereas at low temperature the para form is more stable. Let us learn more about orthohydrogen and the differences between ortho and para and why that is important.
Elementary particles like protons and electrons possess a property called “spin” by looking down on the axis of the particle. Molecular hydrogen consists of two protons and two electrons which can exist in an up or down state, aligned in parallel (see figure), called orthohydrogen or the two protons spin aligned antiparallel, called parahydrogen. This discovery of the forms of hydrogen was led by Werner Karl Heisenberg. In 1932, the Nobel Prize in Physics was awarded to Heisenberg “for the creation of quantum mechanics, the application of which has led to the discovery of the allotropic forms of hydrogen.” These two forms are often referred to as spin isomers or as nuclear spin isomers and exist due to parity between the nuclear spin and the rotational spin function for the molecule. Orthohydrogen exists in a quantum-mechanical state that has higher energy than parahydrogen.
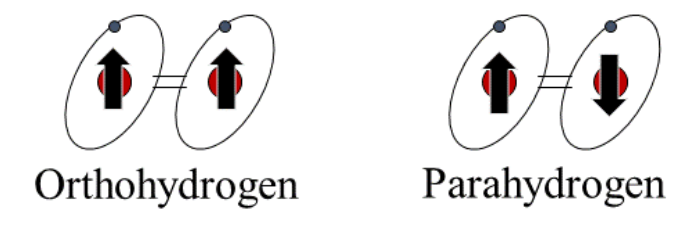
At room temperature and equilibrium, the ratio of ortho to para is 3:1 since orthohydrogen is the more stable form of hydrogen at and above room temperature. Hydrogen of this equilibrium composition is termed normal. At zero kelvin, on the other hand, all the molecules must be in a rotational ground state at equilibrium, therefore all the hydrogen molecules at equilibrium should be in the para state. This characteristic is important and valuable to the use of liquid hydrogen and taking advantage of this ortho conversion to para state.
As the temperature drops, the abundance of parahydrogen molecules increases and at about the liquefaction temperature of air or nitrogen, 77 kelvins (K), the number of ortho to para molecules is about equal (1:1 or 50%/50%), and the transition continues as the temperature drops lower. To produce equilibrium liquid hydrogen, approximately 50% of hydrogen must be converted from ortho to para.
The importance of managing the hydrogen spin state is based on its energy of conversion. Since the energy of conversion (approx. 527 J/g at 20 K) is higher than the heat of vaporization (approx. 447 J/g), nonconverted hydrogen will cause liquid hydrogen to boiloff as hydrogen follows its equilibrium state relative to temperature. Some studies have shown that the majority of the liquid can be evaporated through conversion alone, regardless of heat leak into the tank.
Since the ortho type liquid hydrogen will boil-off much faster, the desired type of liquid hydrogen is para. At GenH2, the goal is zero-loss liquid hydrogen storage units, so the full conversion of ortho to para can happen by pre-conversion using catalysts, by using a controlled storage system, or any combination of the two. Under ambient pressure condition, hydrogen liquefies at approximately 20 K and at this temperature it exists as greater than 99.7 percent para composition. The innovative controlled storage approach allows for keeping the liquid hydrogen in the para state and also controls the absorption of heat from the environment.
Please look for next week’s blog as we cover the alphabet P and discuss Phases and phase changes.
Blog written by GenH2 team member: Martha Williams, Ph.D.



